Bcs Class Iii
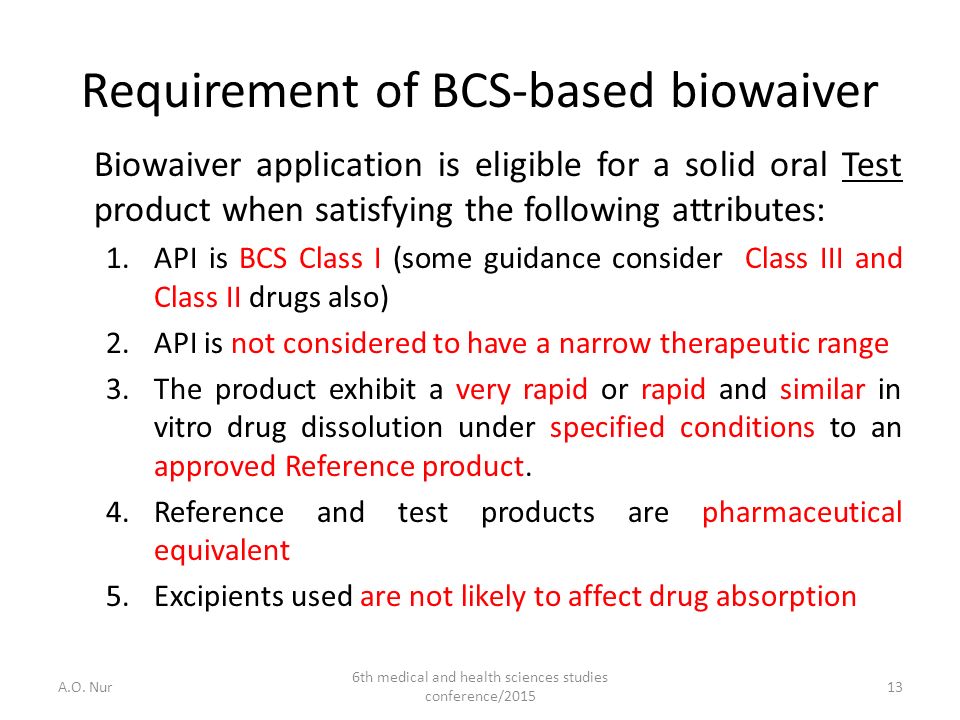
ICH says the guideline, M9 Biopharmaceutical Classification System-Based Biowaivers, will apply only to BCS Class I and III immediate-release, solid oral drugs or suspensions designed to deliver drugs for system circulation. Drugs with a narrow therapeutic index should not considered for BCS-based biowaivers and fixed-dose combination drugs may.
Parr, Alan; Hidalgo, Ismael J; Bode, Chris; Brown, William; Yazdanian, Mehran; Gonzalez, Mario A; Sagawa, Kazuko; Miller, Kevin; Jiang, Wenlei; Stippler, Erika S

2016-01-01
Currently, the FDA allows biowaivers for Class I (high solubility and high permeability) and ClassIII (high solubility and low permeability) compounds of the Biopharmaceutics Classification System (BCS). Scientific evidence should be provided to support biowaivers for BCSClass I and ClassIII (high solubility and low permeability) compounds. Data on the effects of excipients on drug permeability are needed to demonstrate that commonly used excipients do not affect the permeability of BCSClassIII compounds, which would support the application of biowaivers to ClassIII compounds. This study was designed to generate such data by assessing the permeability of four BCSClassIII compounds and one Class I compound in the presence and absence of five commonly used excipients. The permeability of each of the compounds was assessed, at three to five concentrations, with each excipient in two different models: Caco-2 cell monolayers, and in situ rat intestinal perfusion. No substantial increases in the permeability of any of the compounds were observed in the presence of any of the tested excipients in either of the models, with the exception of disruption of Caco-2 cell monolayer integrity by sodium lauryl sulfate at 0.1 mg/ml and higher. The results suggest that the absorption of these four BCSClassIII compounds would not be greatly affected by the tested excipients. This may have implications in supporting biowaivers for BCSClassIII compounds in general.
- Docket Number:
- FDA-2015-D-1245
- Issued by:
Bcs Class 3 Drugs List Pdf
The Food and Drug Administration (FDA or Agency) is announcing the availability of a guidance for industry entitled “Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System.” This guidance finalizes recommendations for sponsors of investigational new drug applications (INDs), and applicants who submit new drug applications (NDAs), abbreviated new drug applications (ANDAs), and supplements to these applications for immediate-release (IR) solid oral dosage forms, and who wish to request a waiver of an in vivo bioavailability (BA) and/or bioequivalence (BE) study requirement. Prince of persia full movie in hindi free download mp4 free.
Submit Comments
You can submit online or written comments on any guidance at any time (see 21 CFR 10.115(g)(5)) Wake up movie 2018.

If unable to submit comments online, please mail written comments to:
The world's greatest fighting game evolves to a whole new level with Ultra Street Fighter IV. Download Street Fighter Iv. Free and safe download. Download the latest version of. Is your PC ready for the return of the definitive beat 'em up? 
Bcs Class Iii
Division of Dockets Management (HFA- 305)
Food and Drug Administration
5630 Fishers Lane, Rm. 1061
Rockville, MD 20852
Bcs Class Iii Rankings
All written comments should be identified with this document's docket number: FDA-2015-D-1245.
Regulated Product(s)